Consolidated CDA Release 2.2 - Local Development build (v2.2). See the Directory of published versions
Consolidated CDA (C-CDA) is a library of CDA templates developed by HL7. It leverages prior efforts from HL7, Integrating the Healthcare Enterprise (IHE), and Health Information Technology Standards Panel (HITSP). It harmonizes that work and consolidates implementation guides developed under the HL7 Health Story Project. C- CDA was originally developed within the ONC’s Standards and Interoperability (S&I) Framework to provide a definitive set of harmonized CDA templates for the US Realm. C-CDA has evolved over time as additional implementer guidance has been developed through the HL7 ballot process to contribute new templates that supplement the available template library. The C-CDA R2.1 implementation guide (IG) is the currently available version of these templates. However, additional IGs have been developed, balloted and published within the C- CDA implementer community to supplement and expand the number of available templates.
The C-CDA Companion Guide (“Companion Guide”) augments guidance provided in C-CDA to improve and expand the exchange of clinical note information through use of HL7 Clinical Document Architecture (CDA) documents. The Companion Guide augments the C-CDA implementation guide to address emerging requirements stemming from new regulations and rising expectations within the implementer community to support interoperability. It provides implementers with additional guidance consistent with accepted best practices that help to drive adoption of additional data elements that are essential for core information exchange use cases in the US. It reinforces certain fundamental information especially relevant to the additional guidance. It exposes implementers to additional guidance and templates available to address the growing need for greater levels of interoperability.
We acknowledge that best practices may in time become certification requirements. Certification requirements should always be confirmed with the certifying organization.
The C-CDA Companion Guide offers a layered approach to conformance requirements. By adopting guidance provided in the C-CDA Companion Guide, implementers can increase their information exchange capabilities as expectations for interoperability expand.
The HL7 CDA R2 (Normative Web Edition 2010) forms the lowest level of conformance requirement. Implementers may reference CDA R2 if they are developing new templates or are seeking to understand a requirement. The HL CDA R2 standard includes extensions that have been defined to meet implementer needs.
C- CDA adds an additional requirement layer on top of the base standard. This may include use of certain available CDA R2 extensions.
Periodically, C-CDA is amended to adjust for technical corrections. The errata releases may introduce additional or updated requirements.
Over time, to keep pace with change, the Companion Guide is updated to keep implementers informed of emerging changes and rising expectations for consistency and greater levels of interoperability. The C-CDA Companion Guide provides insight on emerging conformance specification so implementers can prepare for change, plan for greater consistency, and deliver higher quality C-CDA documents. Conforming to guidance provided in the Companion Guide is optional, but helps implementers prepare for coming changes.
 |
Figure 2: CDA, C-CDA, Errata and Companion Guide Relationships
For example, to determine if a C-CDA document conforms to industry best practices, the following verification steps would be confirmed:
Reference: C- CDA Errata Process; C- CDA Companion Guide and C-CDA Rubric Rules
Throughout the C-CDA Companion Guide, implementer best practice guidance is summarized using a visual conformance block callout like you see below. If the implementer best practice guidance is machine testable, and therefore implementable in the rubric rules supported by validation tools such as the Scorecard tool, then the visual conformance block callout is labeled with “CONF” preceding the identifying number. Otherwise, the conformance block callout is labeled with “BP” preceding the identifying number. Readers cannot rely on the callouts alone to summarize the full range of best practice guidance provided in the Companion Guide.
A C-CDA implementer SHOULD support the standards maturation process to advance the evolution of the C-CDA specification. [BP-001] |
A C-CDA implementation SHALL incorporate all published errata applicable to the templates used. [CONF-002] |
Implementers wishing to create C-CDA documents according to current industry best practices MAY conform to guidance specified in the C-CDA Companion Guide. [BP-003] |
It is important to note that all guidance provided in the C-CDA Companion Guide is considered optional, regardless of the modal verb used.
^13 Available from the HL7 GitHub, https://hl7.org/permalink/?CDAR2.0schema
Reference: C- CDA Errata Process; C- CDA Companion Guide and C-CDA Rubric Rules; Best Practice Guidance for Higher Levels of Interoperability; Guidance Language and Expectations
CDA defines a standard schema, based on the HL7 RIM, for all CDA documents. When there is a need to communicate information where there is no suitable representation in the schema, the CDA standard permits extensions to be developed. These extensions are described in the context of the section where they are used.
The HL7 Structured Documents Work Group (SDWG) maintains a complete list of CDA R2 extensions that are approved for use within the sdtc namespace.^14 The base CDA R2 schema (with approved extensions) can be found on the HL7 CDA Core GitGub repository.^15 The schema/normative folder contains the original published CDA Schema. This is the schema which is published with the base/core standard. This is mainly used for historical reference. The schema/extensions folder contains an SDTC folder which has the updated CDA schema with all SDTC extensions that are approved by HL7.
To perform schema validation on a CDA document instance properly, it is necessary to use the schema that includes the CDA R2 schema extensions. All extensions will use the namespace urn:hl7-org:sdtc. As a document consumer, the possibility of schema extensions needs to be considered.
Reference: CDA Schema, C-CDA Schematrons, Sample Stylesheet
The table below lists the extensions to CDA R2 that have been defined to support requirements in C-CDA.
| Extension [Cardinality] | Definition |
|---|---|
| sdtc:admissionReferralSourceCode [0..1] | This element is a coded concept that represents the type of referral. Its RIM source class is PatientEncounter. Adds to: * componentOf/encompassingEncounter |
| sdtc:alternateIdentification | The alternateIdentification extension provides additional information about an identifier found in the linked role. The extensions augment the id information in the linked role. The id in the alternateIdentification extension SHALL match an id in the linked role. The alternateIdentification provides additional information about a particular identifier, such as its type. As an extension it needs to be safe for implementers to ignore this additional information. * identifiedBy Cardinality is [0..*] * identifiedBy.typeCode = REL * See slide 8 in attached ppt. * POCD_HD000040-alternateIdentifier.xls |
| sdtc:asPatientRelationship [0..1] | Each participant role other than an informant/relatedEntity may have zero or more relationship roles with the patient. Each of these roles can be expressed with an asPatientRelationship element which further describes the type of role using a code element. The informant/relatedEntity participant role already supports specification of the relationship between the informant and the patient via the RelatedEntity classCode, and therefore should not include this extension. (CCD) Adds to: * Person |
| sdtc:birthTime | The sdtc:birthTime element allows for the birth date of any person to be recorded. The purpose of this extension is to allow the recording of the subscriber or member of a health plan in cases where the health plan eligibility system has different information on file than the provider does for the patient. |
| sdtc:deceasedInd [0..1] | The deceasedInd extension is used to record that the recordTarget or subjectPerson is deceased. Adds to: * recordTarget/patientRole/patient * subject/relatedSubject/subject |
| sdtc:deceasedTime [0..1] | The deceasedTime extension is used to record the time of death for the recordTarget or subjectPerson. Adds to: * recordTarget/patientRole/patient * subject/relatedSubject/subject |
| sdtc:desc [0..1] | The desc extension allows multimedia depictions of patients, healthcare providers, or other individuals to be included in a CDA document. It may be used in any person (or derived) entity and appears after the entity name. Adds to: * recordTarget/patientRole/patient * subject/relatedSubject/subject * person |
| sdtc:dischargeDispositionCode | The sdtc:dischargeDispositionCode element allows the discharge disposition to be recorded for an encounter act. |
| sdtc:ethnicGroupCode [0..*] | This ethnicGroupCode extension is used to record additional ethnicity groups for the recordTarget or subjectPerson. Adds to: * recordTarget/patientRole/patient * subject/relatedSubject/subject |
| sdtc:functionCode [0..1] | The sdtc:functionCode extension element allows the function that the participant is doing to be recorded. Adds to: * performer and participant for entries. It currently is available for these data elements in the header and just needs to be added for entry representation. |
| sdtc:id [0..*] | This id extension is used to record the subject’s medical record number or other id. The id extension in the family history organizer on the related subject allows for unique identification of the family member(s). (C-CDA) CDA Release 2.0 does not provide a mechanism to determine when two participants in different roles are in fact the same entity (i.e., an entity can be a person, organization or device). A CDA Document identifies each participant through the application of a role identifier. This identifier can be used to trace the participation of an entity in a given role but cannot necessarily be used to determine that two entities are the same. While more role identities could be provided whose intended use is to unify the entities, this is better modeled through the use of an entity identifier. Therefore, to facilitate this capability, this guide defines an extension to CDA Release 2.0 that allows the person or organization playing the role to be uniquely identified, by the inclusion of an identifier on the entity. Adds to: * subject/relatedSubject |
| sdtc:inFulfillmentOf1 [0..1] | This is an actRelationship called inFulfillmentOf1 that represents the Fulfills General Relationship Operator in QDM 4.1.x in QDM-Base QRDA Category 1, R3 (uses FLFS actRelationship type which is not an allowed actRelationship (entryRelationship) type in CDA). Also create ActReference to contain the pointer to already existing class. Adds to: * Observation * Substance Administration * Supply * Procedure * Encounter * Act Extension will be a pointer (reference) to an already existing order or recommendation. The id of the existing order or recommendation will be used to allow pointing to the already existing data without repeating it in the relationship (ActReference). InFulfillmentOf1 is the relationship between the act that is fulfilling the order/recommendation and that order/recommendation. |
| sdtc:multipleBirthInd [0..1] | The multipleBirthInd extension is used to record that the recordTarget or subjectPerson is part of a multiple birth. Adds to: * recordTarget/patientRole/patient * subject/relatedSubject/subject |
| sdtc:multipleBirthOrderNumber [0..1] | The multipleBirthOrderNumber extension is used to record the order number within a multiple birth that the recordTarget or subjectPerson was born in. Adds to: * recordTarget/patientRole/patient * subject/relatedSubject/subject |
| sdtc:negationInd [0..1] | The Quality Measures need to be able to state that something did not happen and the reason why that thing did not happen. This is accomplished by setting negationInd=”true” and stating the reason (rationale) in a contained Reason template. This is needed for supply and encounters, however CDA has constrained the negationInd out of supply and encounter. (i.e. this device was not supplied because of reason x or this encounter did not happen because of reason y). On 4/23/2015 this proposal was withdrawn. Despite the argument for a consistent approach for negation on all act classes and acknowledgement of the issues unique to negation for observation acts, the proposal was withdrawn based on the requirement in the CDA R2 standard, Chapter 1.4 CDA Extensibility, “These extensions should not change the meaning of any of the standard data items, and receivers must be able to safely ignore these elements. Document recipients must be able to faithfully render the CDA document while ignoring extensions.” Adds to: * Supply * Encounter |
| sdtc:patient [0..1] | The sdtc:patient extension element allows for the patient’s identifier, used by a given provider, to be reported. The provider in their role as an assigned entity is related to the patient. Adds to: * AssignedEntity |
| sdtc:precondition1 [0..*] | The sdtc:precondition1 extension allows for the association of a criterion with a reference range (ObservationRange), which allows the expression in a laboratory report that a reference range is conditional on some criterion such as patient sex or age (or a combination of criterion). Adds to: * observationRange |
| sdtc:priorityNumber [0..1] | The sdtc:priorityNumber extension element allows the priority order of a set of acts to be reported through the use of this element in the component actRelationship of an organizer source act that holds the set of acts being ranked. The RIM states, that priorityNumber is an integer specifying the relative preference for considering this relationship before other like-typed relationships having the same source Act. Relationships with lower priorityNumber values are considered before and above those with higher values. Adds to: * organizer/component |
| sdtc:raceCode | The raceCode extension allows for multiple races to be reported for a patient. Adds to: * recordTarget/patientRole/patient |
| sdtc:raceCode [0..*] | The raceCode extension is used to record additional race codes for the subject. Adds to: * subject/relatedSubject/subject |
| sdtc:signatureText | The signatureText extension adds an attribute for authenticator and legalAuthenticator to record encoded digital signature information. |
| sdtc:statusCode | The statusCode extension attribute allows the implementer to identify a ClinicalDocument that is in other than the completed state. It was created to support the Structured Form Definition IG to identify that the document itself is an unfinished product currently being completed for a patient. |
| sdtc:text [0..1] | The text extension adds the text element to the organizer act. Every other act has a text element, so this was needed to make the organizer act consistent with other acts. It also is needed to support mapping between the organizer act in CDA and the list resource in FHIR. Adds to: * organizer |
| sdtc:valueSet | The valueSet extension adds an attribute for elements with a dataType which indicates the particular value set constraining the coded concept. |
| sdtc:valueSetVersion | The valueSetVersion extension adds an attribute for elements with a CD dataType which indicates the version of the particular value set constraining the coded concept. |
Table 4: CDA R2 Extensions Used by C-CDA
^14 For more information on CDA R2 Extensions, visit https://confluence.hl7.org/display/SD/CDA+Extensions
^15For more information on base CDA R2 schema, visit, https://hl7.org/permalink/?CDAR2.0schema
The C-CDA IG defines templates that specify conformance statements for representing structured clinical notes. CDA templates are defined for document, section, entry, and entry relationship content. Inclusion of a template ID in a CDA document does not convey semantic meaning. A template declaration in a CDA document indicates an expectation that the associated XML conforms to the rules defined by that template.
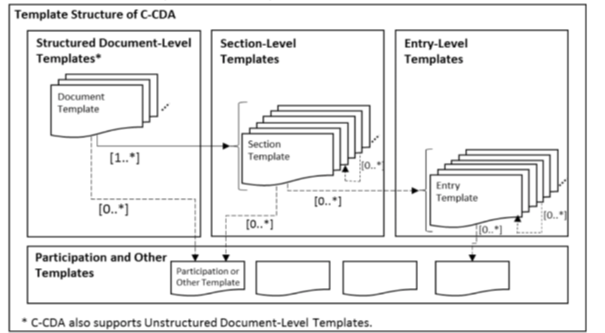 |
Figure 3: Template Structure of C-CDA
CDA documents may declare template conformance at the header level to express conformance expectations for
the content before the
A structured CDA document additionally may declare template conformance at the section level. A template declaration at the section-level establishes conformance expectations for the section itself and for the discrete entries that may be included in the section.
Templates also may be declared at the entry level to express conformance expectations for the discrete data that is represented in the machine processable entry. Template declarations also may be nested within the structural components of an entry to convey conformance expectations about sub-parts of the entry structure.
The presence of a template declaration in a C-CDA document is to define the constraints which stipulate what may, should, or shall be populated in an instance of the document.
Conformance to a template from C-CDA R1.1 (defined prior to the practice of template versioning) is expressed by declaring the templateId of the version of the template published under C-CDA R1.1 in the root attribute with no version information included in the extension attribute.
Schematron is a rule-based validation language for confirming declarations about the presence or absence of patterns in XML trees. Schematron is capable of expressing constraints above and beyond what is possible with XML Schema.
Schematron can be used to:
Each template in the C-CDA library of templates has a corresponding Schematron validation package based upon the conformance constraints defined in the template. This Schematron package can be used to confirm if a CDA document conforms to the constraints required by C-CDA R2.1. The C-CDA Schematron package is updated when errata releases for C-CDA are published. A Schematron package for C-CDA R2.1 is available on the HL7 International Structured Documents Work Group GitHub repository.^16 The C-CDA Schematron packages available at time of publication are included in the Publication Package as a convenience for users. To access the current version of an available Schematron, utilize the versions posted in the GitHub repository.
Reference: Structured Header
C- CDA templates are identified with a templateId. The templateId is a two-part identifier that consists of a root and an extension. The root identifies the named template and the extension identifies the version of that template. Initially C-CDA templates did not include versions. The templateId/@extension attribute was not used. Many of those original templates are still used in C-CDA R2.1.
Chapter 3.1.2 of the Consolidated CDA Implementation Guide discusses the use of templateIds and what needs to be included in a C-CDA document:
(^16) https://github.com/HL7/cda-ccda-2.1/blob/master/schematron/Consolidation.sch
C-CDA R2.1 Content Creators SHOULD NOT declare conformance to irrelevant templates [BP- 004] Note: Testability requires business decisions to be made regarding which templates arenot relevant. |
To avoid confusion and minimize inclusion of unnecessary information in C-CDA documents, implementers should avoid including duplicate or irrelevant templateId declarations.
It is important to note that including the 2.1 templateid and the 1.1 template id is not duplication and is valid to describe the content as conformant to both the 1.1 and 2.1 versions of C-CDA.
Based on emerging implementation expectations for C-CDA based interoperability, a set of criteria are compiled and balloted through HL7 to inform the C-CDA community of additional rubric rules to consider when assessing the quality of C-CDA documents.
The rubric criteria are created through an ongoing project in the HL7 Structured Documents Work Group (SDWG), originating in 2016. HL7 members continually update the rubric which is periodically balloted and then published.
Rubric criteria are created to address key problematic interoperability issues identified in real system-generated C- CDA documents where the community has determined that data is represented inconsistently or incompletely, adversely impacting interoperability. The rubric rules are designed to improve the ability of systems to reliably share and compare data. The goal is to create rubric rules above and beyond the conformance constraints required by CDA and C-CDA to promote best practices by allowing providers and health IT developers to identify and resolve issues in C-CDA documents. Implementers can use the published C-CDA rubric rules to improve data representation in their health IT systems, thereby promoting interoperability and expanding the use of clinical data exchanged in C-CDA documents.
Interoperability is an ever-expanding capability. As new use cases for information exchange emerge and expectations for data sharing rise, additional guidance is needed for implementers to maintain interoperability. Specifications such as C-CDA are published periodically and may not include the most recent developments and growing best practices determined to be needed by the community engaged in information exchange. The C-CDA Companion Guide is produced and published more frequently in order to document and share guidance that has been determined to be essential for interoperability. Best practices documented in the C-CDA Companion Guide inform the C-CDA Content Rubric available for verifying the content of C-CDA documents. The C-CDA Companion Guide also informs the update process used to advance the C-CDA specification. It provides a continuous improvement mechanism for folding essential expanding implementer guidance into the published C-CDA standard.
The following guidance language is not specific to C-CDA templates. It is used in all CDA templates to communicate guidance that applies to creating conformant documents. Conformance statements also are used within the C-CDA Companion Guide to clearly communicate best practice implementer guidance that has emerged as essential for interoperability.
The Companion Guide does not replace or repeat conformance requirements specified in referenced source specifications.
The Companion Guide only provides optional guidance that implementer MAY choose to follow as part of their own path toward higher levels of interoperability. [BP-005] |
In some cases, the Companion Guide provides guidance suggesting that an optional conformance requirement in a source specification should be tightened and become the requirement in order to achieve interoperability. Although the additional guidance suggests a MAY or SHOULD conformance from an underlying specification be elevated to a SHALL, the Companion Guide guidance is still considered optional.
C-CDA documents that do not conform to guidance specified in the Companion Guide SHALL NOT be deemed non-conformant with C-CDA R2.1. [BP-006] |
CDA templates impose constraints based on conformance verbs defined in the HL7 Version 3 Publishing Facilitator’s Guide.^17 Relevant conformance verbs are:
Schematron and other C-CDA Document Validators SHALL indicate Schema and Schematron conformance errors as follows: SHALL violation (error) SHOULD violation (warning) MAY violation (not checked unless present) [CONF-007] |
The table below shows the relationship between conformance verb usage, minimum cardinality and permitted use of nullFlavor.
| Conformance Verb | Minimum Cardinality | nullFlavor Permitted? |
|---|---|---|
| SHALL | 1 | Y (unless explicitly disallowed)^18 |
| SHOULD | 0 | Y |
| MAY | 0 | Y |
Table 5: Conformance Verbs, Cardinality and Use of nullFlavor
^17 http://www.hl7.org/v3ballot/html/help/pfg/pfg.htm
^18 Any SHALL, SHOULD or MAY conformance statement may use nullFlavor, unless the nullFlavor is explicitly disallowed (e.g., through another conformance statement which includes a SHALL conformance for a vocabulary binding to the @code attribute, or through an explicit SHALL NOT allow use of nullFlavor conformance).
Guidance published in the C-CDA Companion Guide is used to develop best practice Rubric which are balloted within the HL7 C-CDA community and adopted as accepted rules for validating content in C-CDA documents. Published C-CDA Rubric are implemented by document validation tools such as the ONC Scorecard to provide feedback on CDA document conformance to the layer of conformance criteria considered by the C-CDA community to be best practice and essential for interoperability.
C-CDA Document Validators MAY support validation of all the layers of conformance described in Chapter 2.3.1 Declaring Template Conformance. [BP-008] |
<br/>
<table>
<tr style="height:49pt">
<td
style="width:468pt;border-top-style:solid;border-top-width:1pt;border-left-style:solid;border-left-width:1pt;border-bottom-style:solid;border-bottom-width:1pt;border-right-style:solid;border-right-width:1pt"
bgcolor="#C5D9F0"><p class="s19"
style="padding-left: 95pt;padding-right: 47pt;text-indent: -54pt;text-align: left;"
>C-CDA Document Validators SHALL indicate best
practice guideline violations as follows: <span
style=" color: #00133A;">SHALL violation
(warning)</span></p><p class="s49"
style="padding-left: 95pt;text-indent: 0pt;text-align: left;"
>SHOULD violation (not checked unless present)</p><p
class="s49"
style="padding-left: 95pt;text-indent: 0pt;line-height: 11pt;text-align: left;"
>MAY violation (not checked unless present) <span
class="s31"><b>[CONF-009]</b></span></p></td>
</tr>
</table>
The following chapters reinforce and explain fundamental information especially relevant to the guidance included in the Companion Guide.
The HL7 Clinical Document Architecture (CDA) is a document markup standard that specifies the structure and semantics of “clinical documents” for the purpose of exchange. A clinical document is documentation of clinical observations and services, with the following characteristics:
A CDA document is a defined and complete information object that can include text, images, sounds, and other multimedia content.^19
^19 HL7 Clinical Document Architecture, R2.0, Normative Web Edition 2010.
Key aspects of CDA include:
CDA documents derive their machine processable meaning from the HL7 Reference Information Model (RIM) and use the HL7 Version 3 Data Types.
Document templates define header requirements as well as section template requirements; they specify a header template as well as section templates as needed. The header template describes the scope and intended use of the document. The header includes the metadata that details contextual information, such as who created the document, encounter information, and patient demographics.
Section templates revolve around a common clinical concept, such as Procedures or Encounters – i.e. the Procedures section template captures information relative to patient procedures.
Section templates are defined globally and may be used by more than one document template. For example, the template defining the Medications section are used in both a CCD and Referral Note. A section template may contain zero, one or many entry templates.
Entry templates represent individual clinical statements through structured data elements, such as a specific medication or procedure. Entry templates may also have requirements for certain data elements to be coded. Entries are very specific templates intended to capture an event, action, or observation relative to the information captured in the section or parent entry. An entry-level template may be used within multiple section-level templates.
Templates are identified using a special Object Identifier (OID). An OID is a globally unique ISO (International Organization for Standardization) identifier. Within the context of HL7 C-CDA, OIDs are represented in the following way: urn:oid:2.16.840.1.113883.10.20.15.3.1 (closed). Often the “urn:oid” is omitted when identifying a specific template.
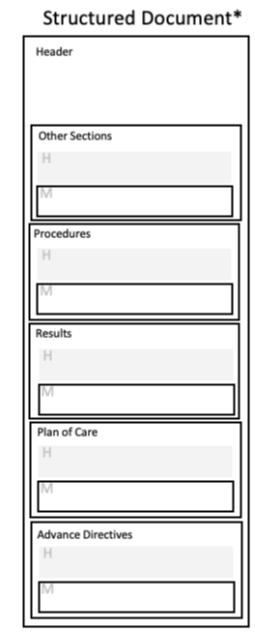 |
For the purposes of this guide, a CDA document with a structured header paired with a structuredBody element will be referred to as a “structured document” and a CDA document with a structured header paired with a nonXMLBody element will be referred to as an “unstructured document”. The structured header for either type of document permits computer processing (parsing) to occur on its content.
Unstructured documents fill an important role where structured information is inappropriate, impractical, or unavailable. Use of unstructured documents facilitates exchange for information that does not yet have standardized representation specifications or when processing of structured data is not yet available at recipient systems.^21
 |
^20 HL7 Clinical Document Architecture, R2.0, Normative Web Edition 2010.
^21 HL7 CDA R2 Attachments IG: Exchange of C-CDA Based Documents, R1 – US Realm, pages 16-19
NOTE: The Unstructured Document template defined in C-CDA does not restrict the type of content that can be represented. Thus, it may prove to be useful for systems with limited capability to create fully structured documents.^22
^22HL7 CDA R2 Attachments IG: Exchange of C-CDA Based Documents, R1 – US Realm, pages 18, 25
Example 1: Pathology Narrative Note A Pathology Narrative Note represented in a minimally structured CDA document which asserts conformance to the C-CDA US Realm Header template and then uses standard LOINC codes for the document type and its narrative sections.
<ClinicalDocument xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance" xmlns="urn:hl7-org:v3" xmlns:cda="urn:hl7-org:v3" xmlns:sdtc="urn:hl7-org:sdtc"> <realmCode code="US"/> <typeId root="2.16.840.1.113883.1.3" extension="POCD_HD000040"/> <!-- US Realm Header --> <templateId root="2.16.840.1.113883.10.20.22.1.1" extension="2015-08-01"/> <id root="2.16.840.1.113883.3.3208.101.1" extension="20130607100315-CCDA-CCD"/> <code code="90371-6" displayName="Clinical pathology Note" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC"/> <title>Pathology Note</title>
…
The rest of the header here.
…
<component> <structuredBody> <component> <section> <code code="81192-7" codeSystem="2.16.840.1.113883.6.1"/> <title>Clinical Pathology Consult Note</title> <text>
…
The narrative note appears here.
… </text>
</section>
</component>
…
Additional narrative sections appear here, if appropriate.
…
</structuredBody>
</component
</ClinicalDocument>
The exchange documents defined in C-CDA use “open templates” in that they permit additional sections to be included when needed to exchange available clinical information.
In open templates, all of the features of the CDA R2 base specification are allowed except as constrained by the templates. By contrast, a closed template specifies everything that is allowed, and nothing further may be included. Open templates allow HL7 implementers to include additional structured content not constrained by the asserted template(s).
At this time, few if any closed templates exist in the specifications referenced by the C-CDA Companion Guide.
HL7 encourages implementers to bring forward use cases that require additional data elements to be included in C- CDA templates. This practice fuels advances in interoperability and maximizes the development of shared semantics as candidate requirements become formalized in subsequent versions of the standard.
As the library of available CDA templates grows and implementers become more experienced using the standard, implementers may use templates developed in other CDA implementation guides which are compatible with C- CDA. Employing additional C-CDA compatible templates will expand the range of interoperable information available for exchange and help address emerging use cases for data exchange.
C-CDA Content Creators MAY include other C-CDA compatible templates defined in other implementation guides. [BP-010] |
A C-CDA compatible template is a template that further constrains a template defined in C-CDA or a template that does not conflict with templates defined in C-CDA. Determining if a template is C-CDA compliant may require human discernment and consensus building within the C-CDA implementer community.
The set of twelve document templates defined in C-CDA R2.1 can be summarized in the following groupings explained in the following chapters.
An encounter summary document is primarily a clinician authored collection of information specific to a single patient interaction with a clinician, care team or hospitalization. The document may be provided to a patient immediately upon, or soon after, the conclusion of their encounter even if all the information related to that encounter is not yet available.^23
Encounter summaries are used to exchange clinical information that was gathered during an encounter with the
patient. The header allows information about the encompassing encounter to be included as structured data,
including who was the responsible party for the rendered care and where the encounter took place. For encounter
summaries, this information SHOULD be included to support emerging use cases for data from C-CDA documents
to support quality measure assessment.
^23 Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Carequality and CommonWell Work Groups. 2018. Page 7.
Patient summaries are used to exchange clinical information about a patient’s care over time. A patient summary is not specific to a particular encounter. The context of the document is a span of time over which care services have been provided.
Other types of clinical information exchange documents used to share information that supports care delivery, planning, and transitions of care.
| Encounter Summary Documents | Patient Summary Documents | Other Categories of Clinical and Patient-Generated Documents |
|---|---|---|
| Consultation Note Discharge Summary History and Physical Note Progress Note |
Continuity of Care Document (CCD) Transfer Summary |
Care Plan Diagnostic Imaging Report Operative Note Procedure Note Referral Note Patient Generated Document |
Table 6: Document templates defined in C-CDA R2.1, sorted by category.
NOTE: Unstructured Documents are classified based upon the document type associated with the LOINC code in the ClinicalDocument/code element in the header of the document.
A great deal of clarity was added in the recent Car e quality CommonWell Joint Document Content Work Group implementation guide to explain the difference between an Encounter Summary document and a Patient Summary document.
An Encounter Summary provides a snapshot of the patient’s condition at the time of the encounter as authored by the clinician. A Patient Summary on the other hand provides a historical view of the information available in the sending system for a span of time which may cross multiple encounters. While the workgroup primarily focused on the importance of creating Encounter Summary documents to complement Patient Summary documents, the work group acknowledged that some systems create CCD documents when requested, based on the IHE XDS Query parameters of the requestor. Systems that support this capability may continue to produce CCD documents in this manner, however, the Joint Document Content Work Group recommends that future development by systems that don’t support this capability focus on implementing Encounter Summary documents, not enhancing CCD generation to match time range parameters of the requestor.^24
Reference: The Joint Document Content Work Group
The HL7 CDA standard was designed to permit information to be exchanged using a document paradigm. The scope of the CDA is the standardization of clinical documents for exchange. The data format of clinical documents outside of the exchange context ( e.g., the data format used to store clinical documents) is not addressed. CDA documents can be transmitted in HL7 messages designed to transfer clinical documents. While the detailed specification for such messages is outside of the scope of the CDA, this specification does impose requirements upon the packaging of CDA documents in HL7 messages. Consult the HL7 CDA standard for additional information about requirements for CDA document exchange.^25
HL7 CDA does not specify the creation or management of documents, only their exchange markup. Document management is critically interdependent with the CDA specifications, but the specification of document management messages is outside the scope of the CDA.^26
In a chapter titled “Smart Senders and Resilient Receivers”, the Car e quality CommonWell Joint Workgroup’s Concise Consolidated CDA implementation guide states:
Successful document exchange relies on layers of rules from CDA document specifications, C-CDA 2.1 specification, and the C-CDA 2.1 companion guide. Despite every effort by implementers, and the HL7 community, to document all the important topics for successful exchange, the Joint Content Work Group discussed many other areas that would benefit from additional guidance. The Smart Senders and Resilient Receivers sections are not an exhaustive list of best practices, but instead is a list of the best practices that captured the group’s attention.
The following chapters summarize best practices identified for C-CDA document Content Creators (Senders) and C- CDA document Content Consumers (Receivers).
Reference: LOINC Coding for Clinical Notes
^24 Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Carequality and CommonWell Work Groups. 2018. Page 23.
^25 HL7 Clinical Document Architecture, R2.0, Normative Web Edition 2010, CDA Document Exchange in HL7 Messages, chapter 3.
^26 HL7 Clinical Document Architecture, R2.0. Normative Web Edition 2010. Scope of the CDA, chapter 1.1.2.
Volume 1 of C-CDA R2.1 includes an explicit requirement to support narrative text linking, which states: The C-CDA R1.1 release recommended that clinical statements include a link between the narrative (section.text) and coded clinical data (entry). Rather than repeat these constraints in every applicable entry, SDWG agreed in C- CDA R2.0 to apply the following constraint to all entry templates, unless explicitly prohibited.
C-CDA Content Creators SHOULD support narrative text linking when creating documents that include sections with discrete data. The primary act of each templated entry: SHOULD contain zero or one [0..1] text (CONF:XXXX). a. The text, if present, SHOULD contain zero or one [0..1] reference/@value (CONF: XXXX). i. This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA R2.0, section 4.3.5.1) (CONF: XXXX). MAY contain zero or one [0..1] originalText (CONF:XXXX). a. The originalText, if present, SHOULD contain zero or one [0..1] reference/@value (CONF:XXXX). i. This reference/@value SHALL begin with a '#' and SHALL point to its corresponding narrative (using the approach defined in CDA R2.0, section 4.3.5.1) (CONF:XXXX). [CONF-011] |
The Joint Document Content Work Group identified this capability as, “extremely important for processing and validating C-CDA documents that include machine-processable entries.” 2018_V1.pdf) The narrative text linkages are the mechanism that associate human readable information in the narrative text of each section to the entries carrying that information for machine processing. Without proper narrative text linking, it is impossible to accurately validate if the machine-readable entries and the human-readable representation of that information accurately reflect the same semantic meaning 2018_V1.pdf).
Resources for more information:
C-CDA Content Creators SHOULD maintain act/observation IDs across documents. [BP-012] |
Many entry templates in C-CDA require an identifier (ID) on an entry.
Reference: Use of Consistent Identifiers
The Joint Document Content Work Group implementation guide states, “Maintaining consistent IDs enables receivers who machine-process documents to de-duplicate the information and accurately identify data that has been previously reported. For any entry where an ID is required, systems SHALL maintain consistent IDs whether sending the entry in an Encounter Summary Document, a Patient Summary document or any other CDA document types.”^32
The C-CDA R2.1 specification does not include a conformance requirement addressing the need for this practice of maintaining act/observation IDs. The Joint Document Content Work Group’s guidance adopts the practice as a strict requirement.^33 This Companion Guide recommends that implementers follow this guidance as a best practice.
C-CDA Content Creators SHOULD support document versioning. [BP-013] |
There are many situations where a document may be updated. For example, a pending laboratory result or a missing note may trigger an update. The base CDA standard provides a mechanism to replace or append a previously sent document through the parentDocument relationship.^34 Since senders will not know what a receiver stored, it is preferable to always send a complete document that replaces the prior document, then indicate the parent document being replaced by including it with the replace relationship (typeCode=”RPLC”).^35
C-CDA Content Creators SHOULD send a complete document and use the replace relationship (typeCode=”RPLC”) when sending a new version of a previously shared document. [BP-014] |
C-CDA Content Consumers SHOULD support replacing a prior version of a document when a document is received that indicates it is a replacement for a prior document. [BP-015] |
Chapter 7 of the HL7 CDA R2 Attachment IG: Exchange of C-CDA Based Documents, R1 – US Realm identifies conformance requirements when using C-CDA documents for attachments shared with Payers.^36 Chapter 7.5 establishes this conformance rule which applies to Content Consumers for handling document succession:
(^31) http://cdasearch.hl7.org/ (^32) Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Car e quality and CommonWell Work Groups. 2018. Page 26. (^33) Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Car e quality and CommonWell Work Groups Chapter 5.1.2 Maintain act/observation IDs across documents. (^34) Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Car e quality and CommonWell Work Groups. 2018. Page 26. (^35) Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Car e quality and CommonWell Work Groups. 2018. Page 27. (^36) https://www.hl7.org/implement/standards/product_matrix.cfm
Document creators SHOULD use the setId and version in the US Realm Header to identify a specific document (document type, patient and visit) the initial version and any successor documents shall use the same setId and increment the version. [AIGEX-DS1] |
C-CDA Content Creators SHOULD use setId and versionNumber to identify document version succession. [CONF-016] |
C-CDA Content Creators MAY indicate section content was reconciled using the Reconciliation Act Entry (1.3.6.1.4.1.19376.1.5.3.1.1.24.3.1). [BP-017] |
The Joint Document Content Work Group implementation guide allows sending systems to indicate that a particular list was reconciled using the IHE Reconciliation Act Entry content module (1.3.6.1.4.1.19376.1.5.3.1.1.24.3.1). The IHE Reconciliation Act Entry defines an entry template to indicate the information in a section has been reconciled.
NOTE: IHE calls CDA templates “content modules”.
The guidance states, “While not required, systems should consider including this act, or a similar indicator, to explicitly state a list has been reconciled.” The guidance also notes, “Only include if the system is confident a user reconciled the list. This should not be included if a clinician simply reviewed the list and did not reconcile it.”^37
C-CDA Content Creators SHOULD include the Section Time Range entry in a section when business logic dictates the range of information that is included. [BP-018] |
Reference: Declaring Business Rules that Limit Section Content; Specifying Time Intervals for Sections with Limits; with Limits
C C-CDA Content Consumers MAY validate documents prior to importing them. [BP-019] |
Chapter 7 of the HL7 CDA R2 Attachment IG: Exchange of C-CDA Based Documents, R1 – US Realm identifies conformance requirements when using C-CDA documents for attachments shared with Payers.^38 Chapter 7.4 establishes these conformance rules which apply to Content Consumers for handling document validation:
All documents SHALL conform to the CDA R2 schema for CDA (XSD) with sdtc extensions included. [AIGEX-VR1] |
All documents SHALL conform to the published HL7 implementation guide conformance specifications for the specific document template (including incorporated section and entry templates) as defined for the specific templateId and extension. [AIGEX-VR2] |
All documents SHALL pass defined as no errors the validation requirements in VR1 and VR2. [AIGEX- VR3] |
Documents that do not meet the validation criteria SHALL NOT be considered a valid attachment for the purpose of this Guide. [AIGEX-VR4] |
(^37) Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Car e quality and CommonWell Work Groups. 2018. Page 28. (^38) https://www.hl7.org/documentcenter/public_temp_E3F202CB-1C23-BA17- 0C6B02F543752C9B/standards/dstu/CDAR2_AIG_CCDA_EXCHANGE_R1_STU_2017AUG.pdf Page 48.
Previously some implementer communities took a different stance suggesting that any C-CDA document that passed CDA Schema validation should, at a minimum, be accepted and systems SHOULD support rendering the section.text information present in the document. The position suggested Content Consumers SHOULD NOT reject documents that did not conform to the C-CDA R2.1 specification for the declared Document templates.
Content Consumers SHOULD be tolerant of accepting non-conformant C-CDA documents because rejecting documents for non-conformance may reduce or delay availability of valuable clinical data. However, Content Consumers may reject non-conformant C-CDA documents. For example, if a document cannot be rendered. Local trading partners may establish additional requirements for accepting documents.
Consumers SHOULD be tolerant of accepting non-conformant C-CDA documents when possible. However, rejecting documents based on an entity’s validation rules or for structural issues may reduce or delay availability of valid clinical data. [BP-020] |
C-CDA Content Consumers SHOULD be able to replace a prior version of a document. [CONF-021] |
Additionally, there are regulatory (Certification) requirements to support sequencing and hiding sections based on a provider’s preferences. A system must allow restricted viewing and support the ability of providers to tailor restricted views as a possible solution for large unusable documents. Use of the C-CDA CDA XSL style sheet will not be sufficient to meet the certification requirements.^39
Content Consumers SHALL support sequencing and hiding sections based on a provider’s preferences and SHALL allow restricted viewing and support the ability of providers to tailor restricted views as a possible solution for large unusable documents. [BP-022] |
The receiving system’s ability to replace a parent document should be maintained regardless of the mechanism of exchange ( e.g. via Direct, query, etc.). Some systems cannot link to prior versions using relatedDocument/parentDocument/id. Due to this inconsistent implementation of linking to parent documents, receiving systems may link to prior versions by using encompassingEncounter/id.^40
Reference: Options for Temporarily Unavailable Data
C-CDA Content Consumers SHOULD use setId and versionNumber to manage document succession.[CONF-023] |
When using C-CDA documents for attachments shared with Payers, additional conformance requirements were set forth in HL7 CDA R2 Attachment IG: Exchange of C-CDA Based Documents, R1 – US Realm. Chapter 7.5 addresses how Content Consumers should handle document succession:
Document recipients SHOULD recognize, associate, and make available versions of documents as defined by the setId and version in the US Realm Header. [AIGEX-DS2] |
Document recipients SHOULD apply any document retention policies to all versions of a document as defined by setId and version.>[AIGEX-DS3]41 |
<br/>
</table><p style="text-indent: 0pt;text-align: left;"
><br /></p><table
style="border-collapse:collapse;margin-left:24.01pt"
cellspacing="0">
<tr style="height:26pt">
<td
style="width:468pt;border-top-style:solid;border-top-width:1pt;border-left-style:solid;border-left-width:1pt;border-bottom-style:solid;border-bottom-width:1pt;border-right-style:solid;border-right-width:1pt"
bgcolor="#C5D9F0"><p class="s19"
style="padding-left: 41pt;padding-right: 8pt;text-indent: 0pt;text-align: left;"
>C-CDA Content Consumers SHOULD display useful
document metadata when showing available documents
for retrieval or retrieved documents.
<b>[BP-024]</b></p></td>
</tr>
<tr style="height:26pt">
<td
style="width:468pt;border-top-style:solid;border-top-width:1pt;border-left-style:solid;border-left-width:1pt;border-bottom-style:solid;border-bottom-width:1pt;border-right-style:solid;border-right-width:1pt"
bgcolor="#C5D9F0"><p class="s19"
style="padding-left: 41pt;text-indent: 0pt;line-height: 12pt;text-align: left;"
>C-CDA Content Consumers SHOULD enable display of
all unrestricted sections of a valid CDA
Document.</p><p class="s31"
style="padding-left: 41pt;text-indent: 0pt;line-height: 12pt;text-align: left;"
>[BP-025]</p></td>
</tr>
</table>
(^39) 80 FR 62634 (^40) Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Car e quality and CommonWell Work Groups. 2018. Page 27.
The Joint Document Content Work Group implementation guide states, “The base CDA standard is designed so that every section’s section.text element is displayable in a basic browser using the base CDA stylesheet, cda.xsl. While receivers are allowed to implement complex processing to apply their own display styles to a section, a system SHALL never hide a section if it does not recognize the LOINC section code. Every properly formatted section SHALL be displayed, or an option given, to allow the user to view the full unrestricted document.”^42
This guidance, combined with the guidance regarding Content Consumer capabilities to limit and customize the rendering of content in a C-CDA document, shows that implementer best practices for Content Consumers includes a wide range of rendering possibilities.
When presenting users with a list of available documents, implementer best practice includes displaying metadata based on guidance included in the Sequoia Project - eHealth Exchange Content Testing Program Guide with the additions of Date and Title by the Joint Document Content Work Group.
Shown below is “Figure 18 Document Information Available during the IHE Query and in the stored C-CDA from the Joint Work Group implementation guide which summarizes key data elements available in the IHE Document Query transaction (ITI-18) and in the C-CDA document header:43
When displaying available documents for retrieval or retrieved documents, systems should display corresponding document information. This information may be obtained from the IHE query/retrieve transaction (i.e., the same as what was displayed in the “list of available documents” during the query) or may be obtained (parsed) from within the C-CDA document header^44.
Document Info |
Availability |
Location |
Date range |
IHE Metadata |
DocumentEntry.serviceStartTime DocumentEntry.serviceStopTime |
Encounter Summary C-CDA Header |
ClinicalDocument/componentOf/encompassingEncounter/ effectiveTime/low ClinicalDocument/componentOf/encompassingEncounter/ effectiveTime/high |
|
Patient Summary C-CDA Header |
ClinicalDocument/documentationOf/serviceEvent/effectiveTime/low ClinicalDocument/documentationOf/serviceEvent/effectiveTime/high |
|
Title |
IHE Metadata |
DocumentEntry.title |
C-CDA Header |
ClinicalDocument/title |
|
Document Type |
IHE Metadata |
DocumentEntry.typeCode |
C-CDA Header |
ClinicalDocument/code |
|
Author |
IHE Metadata |
DocumentEntry.authorPerson |
C-CDA Header |
ClinicalDocument/author/assignedAuthor/assignedPerson |
|
Author Organization |
IHE Metadata |
DocumentEntry.authorInstitution |
C-CDA Header |
ClinicalDocument/author/assignedAuthor/ representedOrganization/name |
|
List of Services |
IHE Metadata |
DocumentEntry.eventCodeList |
Encounter Summary C- CDA Header |
ClinicalDocument/documentationOf/serviceEvent/code |
|
Patient Summary C-CDA Header |
Not Applicable - the service event information in a patient summary is restricted to “Provision of Care”. The document does not contain details about the services provided during the span of time covered by the document. |
|
Practice Type |
IHE Metadata |
DocumentEntry.practiceSettingCode |
Encounter Summary C- CDA Header |
ClinicalDocument/componentOf/encompassingEncounter/ location/healthcareFacility |
|
Patient Summary C-CDA Header |
Not Applicable - Patient Summary may multiple practice types |
|
Format Code |
IHE Metadata |
DocumentEntry.formatCode |
C-CDA Header |
Not Applicable - the formatCode is inferred by the templateIDs asserted in the Header |
Table 7: from Concise Consolidated CDA: Deploying Encounter Summary Documents with Clinical Notes. Document Information Available during the IHE Query and in the stored C-CDA
The meta data mapping provides best practice guidance for use with query/retrieve operations. It also applies to best practices for display of documents received via Direct. Display of these metadata elements offer useful information when selecting documents for retrieval or review.
The term “clinical note” can be used to mean different things, depending on the context of use.
For example, the term “clinical note” can refer to an entire C-CDA document. A C-CDA document is a clinical note in that it includes all the clinical information that is relevant and pertinent to a care encounter, a span of time when care services have been delivered, or a point in time when clinical information about a patient needs to be shared across systems. C-CDA, in fact, was developed to exchange clinical notes in this sense of the term.
Additionally, the term clinical note is often used to describe a document authored by a clinician to capture the health story of a patient – this may include their past and current health as well as planned next steps to improve their health. Clinical notes are a critical part of the patient record. Prior to the formation of the Joint Document Content Work Group the independent Car e quality and CommonWell content work groups were discussing methods to exchange clinical notes in C-CDA. Additionally, in response to requirements within the 21st Century Cures Act, to identify a common set of data for exchange, the Office of the National Coordinator (ONC) has included Clinical Notes in U.S. Core Data for Interoperability (USCDI). The exchange of clinical notes is also a high priority for the further development of the Fast Healthcare Interoperability Resources (FHIR) specification as supported through the Argonaut Project.^45
Within EHR systems, a clinical note may refer to narrative information that is entered by a clinician in a patient’s record. This type of clinical note is clinical information that is not captured as structured data. It exists within the context of a patient’s record and is part of the documentation gathered during an encounter or related to a specific care event. A clinical note of this type could be a single sentence to record an impression, or it could be a paragraph of information that tells a larger story. It is part of the larger clinical note produced to tell the whole story of the encounter.
Regardless of the granularity, clinical note information can be categorized by the type of information contained in the note. Several common categories of clinical notes exist to help classify and organize much of the clinical information exchange today. The categories can apply to collections of information gathered as a whole document, a Notes Section within a larger document, or a single Note Activity entry that holds a narrative clinical note that appears within a standard structured section.
A clinical note created and managed in an EHR system may be represented for exchange with other systems using a C-CDA document template designed for that type of clinical note. The document templates defined in C-CDA establish the section structure for several common types of structured clinical notes. Not all systems used by practitioners to generate clinical notes maintain internal information structures sufficient to classify clinical note information into the structured sections defined for C-CDA structured documents.
To address the challenge that clinical notes in an EHR systems have varying levels of identifiable structure, C-CDA defines clinical note templates at different levels.
Clinical note information that can be represented as a complete document using the section structures required and recommended by C-CDA document-level templates may be exchanged as a structured clinical note document.
Clinical note information which does not contain all the structure required for a C-CDA document may be represented within a section-level template in the context of a larger collection of information pulled together to complete the content required by the document-level template. A Notes Section is used when the type of clinical note information that needs to be shared isn’t specifically aligned with one of the standard sections defined for the document.
An entry-level template called the Note Activity template is defined to represent narrative clinical note information from the originating system. A Note Activity entry is used to represent clinical note information within a document section in a machine processable format.
This layered approach to representing clinical notes in C-CDA enables the wide range of information gathered in EHR systems to represented and shared via a CDA Document. Narrative note information as well as discrete data can be included and encoded in a structure way that facilitates both human readability and machine processing.
Reference: Clinical Note; The Joint Document Content Work Group; Appendix A: Templates Defined in C-CDA R2.1 Companion Guide
Logical Observation Identifiers Names and Codes (LOINC) is an international standard code set developed and maintained by the Regenstrief Institute for identifying clinical information.^46 Since its inception, Regenstrief has developed LOINC as an open standard and is available at no cost. LOINC is used worldwide for the exchange and pooling of clinical results for care delivery, outcomes management, public health reporting, document management, and research. Used in conjunction with standards for messages, documents, and APIs, LOINC supports efficient processing and storage of data from disparate sources. When exchanging clinical information between providers and with payers, attachment requests and attachment submissions use LOINC codes to identify the type of information desired and the information provided.^47
The LOINC terminology includes thousands of different clinical note types.^48 These codes can be used at the document, section, or entry level to categorize the type of clinical note information being shared. To focus the industry, the Argonaut participants and the Department of Veterans Affairs contributed their most commonly used note types to develop the following list of most frequently created clinical note documents.^49 The table below includes the clinical note type, the most general LOINC code available for this type of document, and the value set listing the full range of LOINC codes available for the clinical notes of that type.
The clinical note type value sets are established by the HL7 Payer/Provider Information Exchange Work Group. The value sets use LOINC document codes, are maintained by Regenstrief Institute, and are published through the US National Library of Medicine Value Set Authority Center (VSAC).^50 Each value set is identified by a unique object identifier (OID). These value sets are included in the C-CDA value set expansion package available as a VSAC downloadable resource.^51
| Clinical Note Type | Most General LOINC Code | LOINC Long Name | Complete Note Type Value Set |
|---|---|---|---|
| Discharge Summary Note | 18842-5 | Discharge summary | DischargeSummaryDocumentType 2.16.840.1.113883.11.20.4.1 |
| Consultation Note | 11488-4 | Consult Note | ConsultDocumentType 2.16.840.1.113883.11.20.9.31 |
| Imaging Narrative Note | 18748-4 | Diagnostic imaging study | CompleteNoteType 1.3.6.1.4.1.12009.10.2.5 |
| Laboratory Narrative Note | 11502-2 | Laboratory report | Note Types 2.16.840.1.113883.11.20.9.68 |
| Pathology Narrative Note | 11526-1 | Pathology study | Note Types 2.16.840.1.113883.11.20.9.68 |
| History & Physical Note | 34117-2 | History and physical note | HPDocumentType 2.16.840.1.113883.1.11.20.22 |
| Progress Note | 11506-3 | Progress note | ProgressNoteDocumentType 2.16.840.1.113883.11.20.8.1 |
| Procedures Note | 28570-0 | Procedure note | ProcedureNoteDocumentType 2.16.840.1.113883.11.20.6.1 |
Table 8: LOINC Coding for Document Level clinical note types
The list was not provided in a priority order, nor was it intended to represent the exclusive list of what systems can and will support. Guidance from the Joint Document Content Work Group encouraged support for C-CDA document templates of these clinical note types.
C-CDA Content Creators SHOULD support creation of C-CDA documents for multiple clinical note types.[BP-002] |
Guidance from the Joint Content Document Work Group also encouraged support for the Notes Section template which covers the standardized clinical note sections for use in C-CDA structured documents shown below.
The table below includes common categories of clinical note information. These LOINC codes are used to identify a section that includes narrative clinical notes of this type. These LOINC codes also are used to identify an individual clinical note when it is included as a machine processable entry within a standard C-CDA section. The Note Types value set (2.16.840.1.113883.11.20.9.68) identifies the full set of LOINC Codes that can be used with the Notes Section and the Note Activity entry.
| Type of Narrative Clinical Note Information | LOINC Code for Note Section and/or Note Activity Entry |
|---|---|
| Consultation Note | 11488-4 |
| Discharge Summary Note | 18842-5 |
| History & Physical Note | 34117-2 |
| Imaging Narrative Note | Appropriate LOINC Code with Scale = DOC |
| Laboratory Narrative Note | 11502-2 |
| Note | 34109-9 |
| Nurse Note | 34746-8 |
| Pathology Narrative Note | 11526-1 |
| Patient Note | 51855-5 |
| Procedures Note | 28570-0 |
| Progress Note | 11506-3 |
| Referral Note | 57133-1 |
| Surgical Operation Note | 11504-8 |
| Transfer Summary Note | 18761-7 |
Table 9: LOINC Codes for section- and entry- level clinical notes
C-CDA Content Creators SHOULD support inclusion of narrative clinical note information in structured sections of C-CDA documents.[BP-027] |
Reference: Sections Defined in C-CDA (ordered using SOAP framework); Clinical Note;
There may be situations when information is not available at the time a CDA document is created. In these cases, a document containing available information may be sent. If the document type being sent requires a section for which the information is not yet available, the required section should be coded at the section-level to indicate the information for that section is “not available” using the ”null”Flavor=”NAV” attribute. If the document type being sent indicates the section for which information is not yet available is an optional section, then inclusion of that section is not needed.
At a later point in time, when the information becomes available to complete the document, a new version of the document may be created and marked to communicate that it supersedes the previous version of the document. Specifically, the new document is identified with a globally unique identifier in the clinicalDocument.id field. The relatedDocument/typeCode=“RPLC” and the relatedDocument/typeCode=”RPLC”/parentDocument/id element will be set to reference the prior document’s clinicalDocument.id.
The setId and versionNumber fields are only used to indicate that the new document is not considered a new document, but rather is considered a new version of a prior document. One use case for using issuing a new version of an existing document would be when correcting an error in the content of the document. Another example might be when distributing the same document to more or different information recipients. Business rules determine the circumstances when a document instance is a new document or a new version of an existing document.
An example includes the requirement of a Hospital Course section within a Discharge Summary. Typically, this section is not available at the time of a hospital discharge. However, but the Discharge Summary document type may still be used to meet the objective for transmitting health information within 36 hours of the hospital discharge. In this example, the incomplete Discharge Summary may be sent at the time of discharge and a new Discharge Summary may be sent later to communicate the updated information.
Example 2: Discharge Summary with no Hospital Course information (see replacement document below).
<ClinicalDocument xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance" xmlns:sdtc="urn:hl7-
org:sdtc" classCode="DOCCLIN" moodCode="EVN" xmlns="urn:hl7-org:v3">
<realmCode code="US" />
<typeId root="2.16.840.1.113883.1.3" extension="POCD_HD000040" />
<templateId root="2.16.840.1.113883.10.20.22.1.1" extension="2015-08- 01" />
<templateId root="2.16.840.1.113883.10.20.22.1.8" extension="2015-08- 01" />
<id root="2.16.840.1.113883.19.5.99999.1" extension="20160414014447" />
<code codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC" code="18842-5"
displayName="Discharge Summary" />
<title>Health Summary</title>
<effectiveTime value="20160414014447-0500" />
<confidentialityCode codeSystem="2.16.840.1.113883.5.25" code="N" />
<languageCode code="en-US" />
<setId extension="20160414014447" root="2.16.840.1.113883.19.5.99999.19" />
<versionNumber value="1" />
...
<section nullFlavor="NAV">
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.5"/>
<code code="8648-8"
displayName="HOSPITAL COURSE"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<title>Hospital Course</title>
<text>Information Not Available</text>
</section>
...
</ClinicalDocument>
Example 3: Replacement Discharge Summary document with Hospital Course information.
The addition of Hospital Course information is not an errata correction, so it does not generate a new version of the document. The new document is indicated to wholly replace the prior document.
<ClinicalDocument xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance" xmlns:sdtc="urn:hl7-
org:sdtc" classCode="DOCCLIN" moodCode="EVN" xmlns="urn:hl7-org:v3">
<realmCode code="US" />
<typeId root="2.16.840.1.113883.1.3" extension="POCD_HD000040" />
<templateId root="2.16.840.1.113883.10.20.22.1.1" extension="2015-08- 01" />
<templateId root="2.16.840.1.113883.10.20.22.1.8" extension="2015-08- 01" />
<id root="2.16.840.1.113883.19.5.99999.1" extension="20160414145050" />
<code codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC" code="18842-5"
displayName="Discharge Summary" />
<title>Health Summary</title>
<effectiveTime value="20160414145050-0500" />
<confidentialityCode codeSystem="2.16.840.1.113883.5.25" code="N" />
<languageCode code="en-US" />
<setId extension="20160414014447" root="2.16.840.1.113883.19.5.99999.19" />
<versionNumber value="2" />
<relatedDocument typeCode="RPLC">
<parentDocument>
<id root="2.16.840.1.113883.19.5.99999.1" extension="20160414014447" />
<code codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC" code="18842-5"
displayName="Discharge Summary" />
<setId extension="20160414014447" root="2.16.840.1.113883.19.5.99999.19" />
<versionNumber value="1" />
</parentDocument>
</relatedDocument>
<section>
<templateId root="1.3.6.1.4.1.19376.1.5.3.1.3.5"/>
<code code="8648-8"
displayName="HOSPITAL COURSE"
codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC"/>
<title>Hospital Course</title>
<text>The patient was admitted and started on Lovenox and nitroglycerin paste. ...</text>
</section>
</ClinicalDocument>